Water chemistry and understanding the pH during the brewing process will help you make impressive quality beers consistently.
What effect does pH have on brewing?
The correct pH will help carry flavour and ensure optimal enzymatic activity. It would help if you had the correct temperatures for enzyme activity. Still, it’s equally important to have the correct ph. Hence, Geterbrewed has added a new range of pH papers and pH meters to help you make better-quality beer. Our tip for understanding pH is to take regular readings at different stages through the brewing process so you can understand what’s happening.
Learning how to measure pH. What are the options?
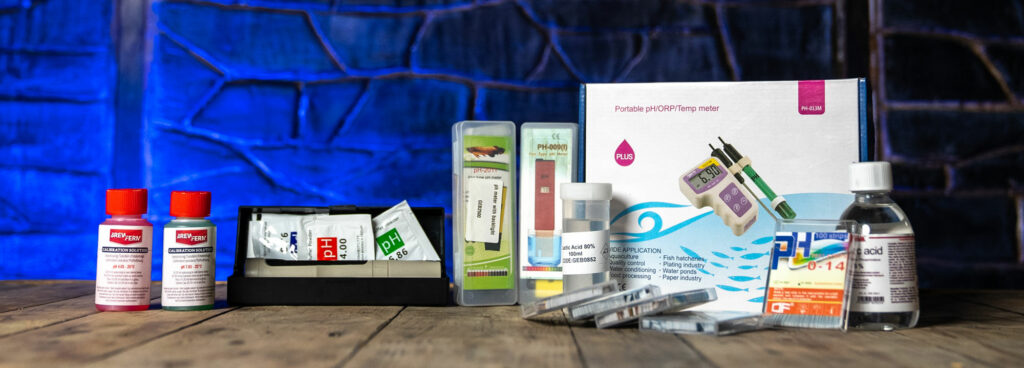
(1) Use Professional Quality pH Papers
Using professional quality ph paper, soaking a test stripe for half a second is all it needs. Then you compare it to colour grading on the packet for an instant visual result. Geterbrewed stock a selection of ph papers with precision accuracy.
(2) Use a pH Meter
Taking a sample for a pH meter is simple. However, you must ensure the pH meter is calibrated before you begin. Geterbrewed stock a wide range of pH meters suitable for different budgets, with different features to suit your needs.
How to operate a portable pH meter.
- Connect the pH- electrode and temperature probe to the probe inset of the instrument
- Remove the protective cap of the pH electrode
- Turn on the meter
- Immerse the ph electrode and temperature probe in the solution to be tested. Stir gently and wait for the reading to stabilise
- After use, turn off the meter. Rinse the electrode with tap water to minimise contamination. Replace the protective cap on the pH electrode

How to calibrate a pH Meter.
- Pour a small quantity of the buffer solution into two clean beakers
- For remarkably accurate calibration, it is advised to use two beakers for each buffer solution, the first is to be used for rinsing the electrode, and the second is used for calibration. Doing it this way eliminates the risk of contaminating the buffer solution
- Switch on the instrument
- Press the pH key to display the ph measurement
- Immerse the electrode in a pH 6.86 buffer solution and gently mix
- Allow the reading to stabilise with a small screwdriver. Turn the calibration trimmer at the bottom of the instrument until the display matches the corresponding buffer/calibration solution. Then rinse the electrode with distilled water.
- Immerse the electrode in the pH 4.01 buffer solution and gently mix
- After approx one minute, with a small screwdriver, turn the calibration trimmer at the bottom of the instrument until it matches the corresponding buffer solution.
- The Calibration is complete – this will need to be repeated regularly. We suggest at least once per month.
What range of pH are you looking for in brewing?
This will change from beer style to beer style. Converting the starch in the malt to fermentable sugars will occur at optimal ph levels. The commonly used mash pH range will be around 5.2 to 5.8pH. We would suggest that a strong wort would be lower and a weak wort higher. The last running will also have a higher pH and can be stopped with regular hydrometer checks, cutting off into the kettle at 1006.
Your finished beer should be at 4.5pH. If adding fruit or doing sours, this will be significantly lower. also, be aware that dry hopping will affect the beer’s ph.

How do you adjust mash pH?
A tip before you take your mash ph is to make sure it is sufficiently mixed and given a few minutes before you take the reading. Also, be aware that you may want to use your recipe calculator to adjust the pH before you start; based on a collection of water data and ingredient data. You want to take your reading at a stable temperature, not the actual mashing temperature, unless the meter you’re using can take and adjust to different temperatures.
There are several products available to adjust pH, for example:
- Phosphoric Acid & Lactic Acid are commonly used to decrease the pH.
- Calcium and magnesium cause a desirable pH lowering, while carbonate and bicarbonate have an adverse effect.
- Certain enzymes, particularly alpha-amylase, are stabilised by calcium.
Other things to consider:
- The essential ions for beer flavour are sulphate (gives dry flavour), chloride (full and sweet), magnesium (bitter/sour) and sodium, and to a lesser extent, potassium (salt flavour).
- Beers with a high pH are prone to infection by microorganisms.
- Variations in beer pH can reflect inconsistency in fermentation.
Check out our YouTube video on pH Testing: